Our Businesses
Exothermic welding
Discover the principles of exothermic welding
Principle of exothermy
Aluminothermy is a chemical principle which allows the production of high temperatures, by a reaction known as exothermic, i.e. it produces heat. This reaction is obtained on metals by means of powdered aluminum, and is triggered by the combustion of a wick of magnesium, which is set on fire by a spark or a flame. Once started, this process cannot be stopped, and must be completed by the complete combustion of the metals used. The addition of water, or even complete immersion, cannot oppose this reaction.
The temperature obtained is higher than the melting temperature of the metals used and approaches their boiling temperature. Thus it can reach 1800 to 3500°c depending on the metal or alloy exposed to the reaction.
Aluminothermy allows the fusion between metals, this property is used in particular for industrial applications.
Use of aluminothermy as a process for extracting metals from ores
Many ores exist naturally in the form of oxides. For example, iron, zinc, chromium, manganese, vanadium, etc. It is necessary to process them to extract metals that are used in industry. One of the possible processes is aluminothermy, and more precisely the oxidation-reduction generated by this technique. It is also called redox reaction.
It is a chemical reaction which consists in a complete transfer of the oxygen molecules (and thus of the oxidation) of the metal subjected to the reaction, towards aluminium.
Le minerai est mélangé à de la poudre d’aluminium dans des proportions bien déterminée, et une combustion est créée au moyen d’une mèche en magnésium, ce qui déclenche la réaction.
Il se produit une fusion au cours de laquelle l’oxygène est capté par l’aluminium. A la fin du processus, le minerai laisse place à un métal pur, et l’aluminium est oxydé.
In this process, the aluminum is called "oxidizing", the ore that gives up its oxygen is called "reducing".
Example of weldable metals and alloys, and their melting and boiling temperatures:
- Brass: 900°c / 2300°c (approx.)
- Bronze : 890°c / 2250°c (approx.)
- Copper: 1084°c / 2567°c
- Stainless steel : 1400°c / 2600°c (approx.)
- Steel: 1450°c / 2650°c (approx.)
- Cast iron : 1820°c / 3000°c (approx.)
- Iron : 1535°c / 2750°c
NB: For alloys, these temperatures vary according to the carbon content and other components.
A precise dosage is sought for an optimal aluminothermic reaction, depending on the metal used. In the case of iron, its ideal proportion will be 74.7%, to which 25.3% of aluminium powder will be added.
Use of aluminothermy for its oxidation-reduction properties
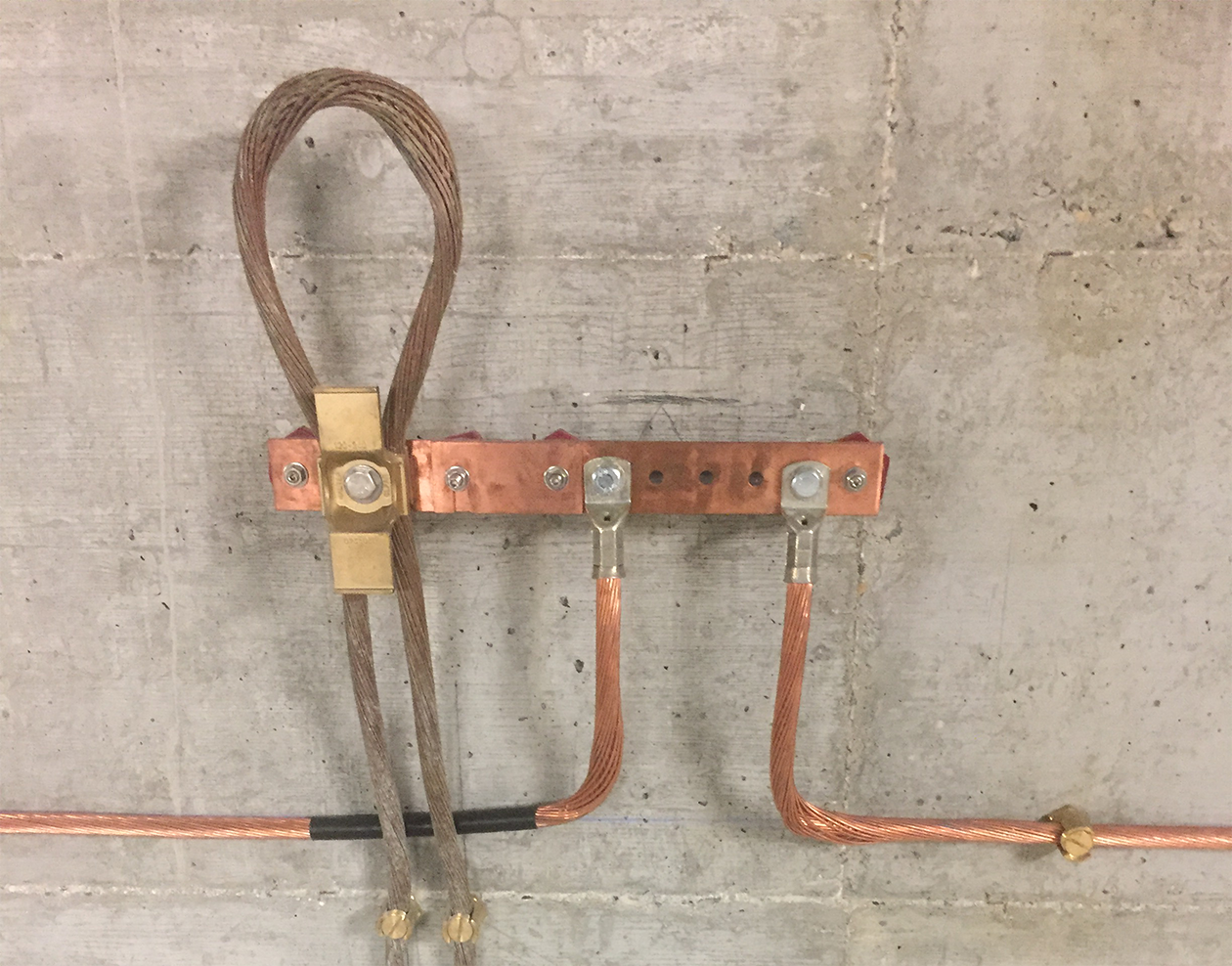
In addition to this property of extracting metal from an ore, the oxidation-reduction that accompanies the aluminothermic reaction allows the return of an oxidized metal to its initial pure form, by the same principle as that described above. Thus, aluminothermic welding allows, for example, to weld a copper cable on a steel post, even if both elements are strongly oxidized. During the reaction, the oxygen contained in the rusted metal will be captured by the aluminum. The metal will then regenerate, allowing a perfect assembly without any trace of oxidation altering the conductivity.
This redox application is useful for all welds on metals located outdoors and exposed to the weather, where good conductivity is required, such as :
Cables, straps, copper plates, grids, flat or round tinned copper braids, stainless steel or copper plated ground stakes, metal beams.
These items are available from Maltep, and are used for grounding or bonding connections, lightning protection, connections to structural steel, etc.
They concern several trades, including: electricians, structural steel carpenters, roofers, and potentially all building and public works professions.
What is exothermic welding?
Exothermic welding is an assembly process by molecular fusion of two metallic parts, by means of a chemical reaction called aluminothermy.
This technique is mainly known for its use in railroad track connections, but it is also applied in shipbuilding, in electrical transformers and wiring of high and medium voltage lines, for cathodic protection, and in metallic construction in general.
It allows a high quality assembly, almost indestructible, free of porosity, and very resistant to oxidation. The parts are amalgamated in a totally homogeneous way. It is a technique that allows particularly reliable and long-lasting welds.
In addition, exothermic welding benefits from outstanding general properties, such as:
- High conductivity, resistance to current surges and short circuits.
- Excellent mechanical resistance.
- Remarkable resistance to the saline atmosphere.
The implementation of the process does not require any energy other than that produced by the chemical reaction itself. Welding on site is therefore completely autonomous.
The qualities of aluminothermic welding allow it to be used, among other things, for the connection of all bare cables, particularly those dedicated to earth connections, ground connections and lightning protection.
MALTEP offers all the necessary equipment to carry out, in a simple and accessible way, aluminothermic welding of electrical earthing cables, lightning conductors in flat or round form, or to connect an earthing conductor to a metal frame or structure.
How to make an electrical connection by exothermic welding?
To perform a exothermic weld, you must first gather the following elements:
- a graphite mold adapted to the components to be welded (cable / flat / ground rod / structure / other)
- the filler metal (welding powder and ignition powder)
- an igniter
- a card cloth brush
- a mold scraper
- a mold cleaning brush
- a clamp adapted to the size of the mold
- sealing compound for certain configurations
MALTEP offers all the necessary equipment to carry out, in a simple and accessible way, exothermic welding of electrical earthing cables, lightning conductor down conductors in flat or round form or even for the connection of an earthing conductor to a metal structure or framework.
Process
After carefully cleaning the materials to be welded, place the mold with the clamp and heat it with a blowtorch or a welding lamp to remove all traces of moisture.
Then place the conductors to be welded, making sure that the mold is perfectly sealed. For this purpose, use sealing compound, if necessary. Place the metal disc at the bottom of the mold crucible. Then pour the filler metal, then on top of it the ignition powder, leaving a part of it overflowing at the edge of the mold, in order to favour the ignition.
Close the mold and cause a spark from the igniter to the ignition powder. Keep clear during burning, and wear protective equipment throughout the operation, including gloves and a visor.
About one minute after the end of the burn, reopen the mold and clear it of slag, using the scraper, cloth brush, and paintbrush.
The welding is finished and the mold is reusable, as long as it is always clean and dry.
Find all the exothermic welding equipment at MALTEP
MALTEP MALTEP puts at your disposal all the parts necessary for the exothermic welding of cables, straps, flat bars, metal frameworks, rails, reinforcing bars and earth rods:

Welding powder
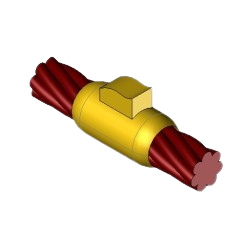
CC1 Exothermic molds
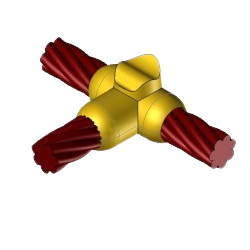
CC2 Exothermic molds
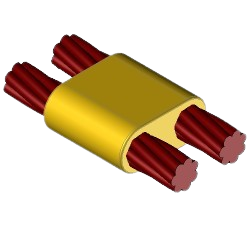
CC14 Exothermic molds
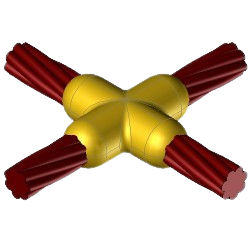
CC4 Exothermic molds
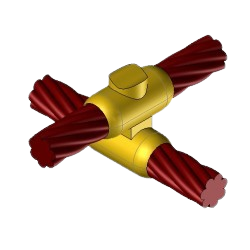
CC11 Exothermic molds
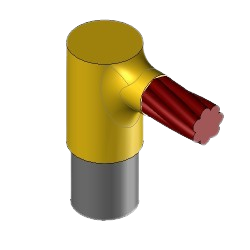
CR1 Exothermic molds
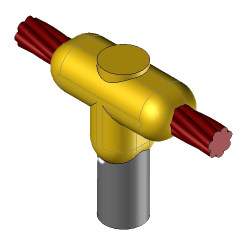
CR2 Exothermic molds
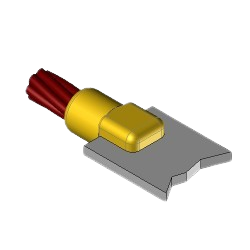
CB1 Exothermic molds
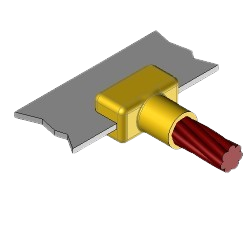
CB4 Exothermic molds
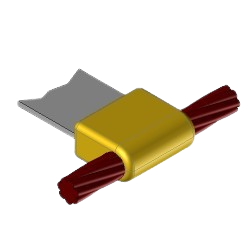
CB5 Exothermic molds
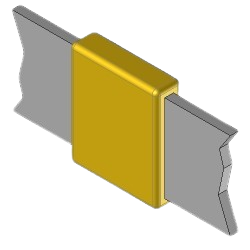
BB1 Exothermic molds
BB7 Exothermic molds
BB14 Exothermic molds
BB41 Exothermic molds
BS1 Exothermic molds
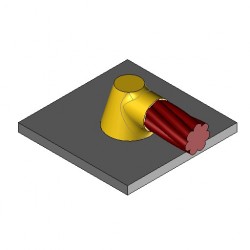
CS1 Exothermic molds
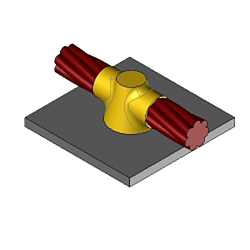
CS2 Exothermic molds
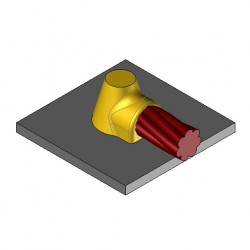
CS8 Exothermic molds
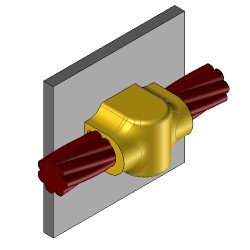
CS27 Exothermic molds
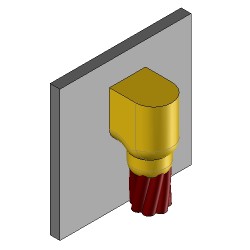
CS25 Exothermic molds
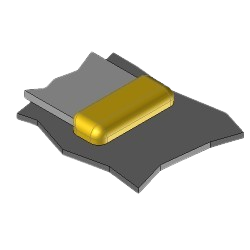
BS2 Exothermic molds
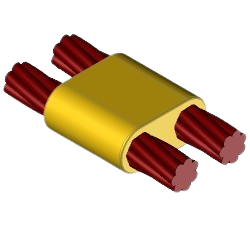
CC14 Welding kit
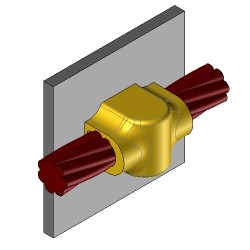
CS27 Welding kit
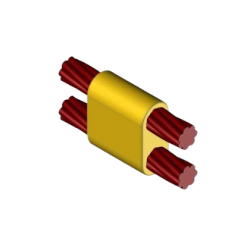
CC7 Exothermic molds
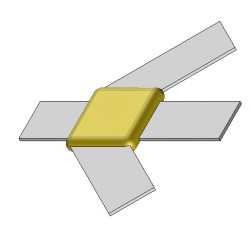
BB47 Exothermic molds
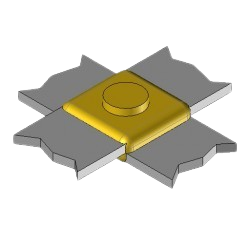
BB40 Exothermic molds
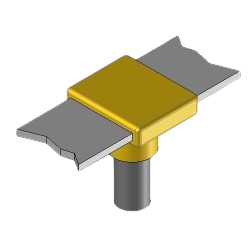
BR30 Exothermic molds

Flint ignitor
Handle clamps

Vise grip plier

G clamp